
News and Announcements from the CDB
Insulin/insulin-like growth factor (IGF) signaling plays an important role in the regulation of biological processes such as growth, metabolism, reproduction and longevity, and is known to be widely conserved across species. In mammals, major players of this pathway are insulin and IGF, which have distinct roles in metabolism and growth, respectively, whereas in the Drosophila fruit fly, there are eight insulin-like peptides (Dilps) that fulfill these roles. The function and regulation of these endocrine hormones must be capable of readily adapting to environmental changes, particularly to nutrient availability, but the mechanisms by which organisms can sense these changes to regulate hormone levels remain poorly understood.
In new work published in the journal Developmental Cell by former research scientist Naoki Okamoto and colleagues in the Laboratory for Growth Control Signaling (Takashi Nishimura, Team Leader), they examine the function of nutrient-dependent growth control hormone, Dilp5, and the mechanisms regulating its production in Drosophila. They pieced together their findings to reveal a complex signaling relay and positive feedback mechanism at work to control the expression of dilp5 in the insulin-producing cells of the fly brain. Okamoto has since moved to the University of California, Riverside, to continue his research.
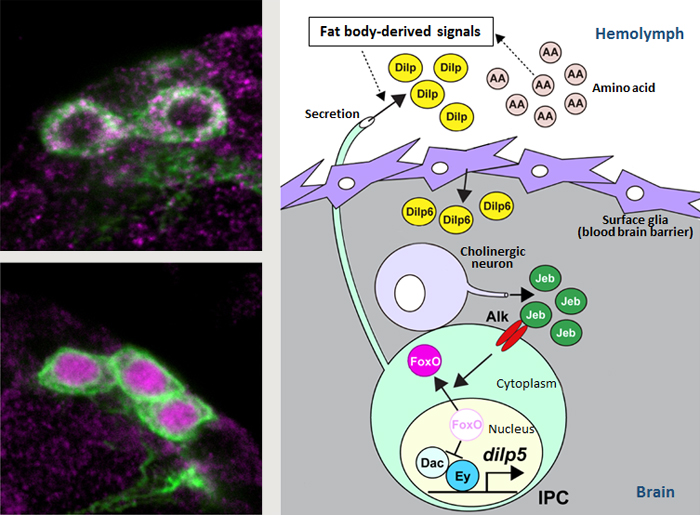
Left: FoxO in IPCs is shuttled from cytoplasm to the nucleus under starved conditions (green, IPC (dilp2); pink, FoxO).
Right: Model of the regulatory mechanisms of nutrient-dependent dilp5 expression.
In Drosophila, Dilps are primarily secreted by insulin-producing cells (IPCs) in the brain in response to a signal from the fat body (insect equivalent of vertebrate liver) which acts as a sensor of nutrient availability. The secreted Dilps then circulate throughout the body to act on target tissues. One of the main Dilps produced by IPCs is Dilp5, an important regulator of body growth. Expression levels of dilp5 are initially low immediately after larval hatching, but rise sharply as larvae begin to feed, and expression levels are also known to fluctuate depending on availability of nutrients in the surrounding environment. A past study by Okamoto and his colleagues demonstrated that dilp5 expression was stimulated through the synergistic action of two transcription factors, Eyeless (Ey) and Dachshund (Dac), in IPCs (*Science News: February 2, 2012). However, it remained unclear how Ey and Dac were tied to nutrient-dependent changes in dilp5 expression.
In this study, the team first tested different nutrient mixtures to determine the critical nutrient source regulating dilp5 expression was amino acids. Target of rapamycin (Tor) signaling, a well-known amino acid sensing pathway, was analyzed as a potential upstream signal regulating IPCs, but the disruption of various factors in this pathway showed no effects on dilp5 expression. They shifted their focus to search for a nutrient-dependent molecule that could regulate the Ey-Dac protein complex, and transcription factor FoxO surfaced as a prime candidate because its activity is known to be regulated by nutrient availability, and can both activate and downregulate expression of a diverse array genes. Starvation experiments revealed FoxO localization moved from cytoplasm to nuclei of IPCs, and that nuclear FoxO can interact directly with Ey, thereby competing with Dac for binding to Ey. Thus, localization of FoxO within the cell can switch expression of dilp5 on and off.
How then is FoxO localization regulated? They screened for potential signaling factors in the IPC to uncover the activation of anaplastic lymphoma kinase (Alk) leads to FoxO localization in the cytoplasm and promotes dilp5 expression. Alk was in turn found to be activated by a secreted ligand called Jelly belly (Jeb), discovered to be produced by cholinergic neurons. Thus, Jeb secreted by cholinergic neurons surrounding the IPC regulated FoxO localization indirectly. Further experiments showed that Dilp6 produced by surface glial cell layer of the Drosophila central nervous system, which functions similarly to the blood brain barrier of mammalian brain, was responsible for regulating Jeb. Surface glial cells are exposed to the hemolymph, and IPC-derived Dilps (Dilp2, 3, 5) released and circulating in the hemolymph stimulate dilp6 expression in glial cells, initiating the indirect signaling relay to enhance dilp5 expression in the IPC through a positive feedback loop.
“It’s puzzling why Drosophila use such a complicated mechanism. Under poor food conditions, dilp5 mutants showed reduced growth rates and smaller adult body sizes than wildtype, and required longer time for larval development, suggesting that the multistep dilp5 regulatory mechanism is important to maintain insulin signals needed for promote growth, even under nutrient-restricted conditions,” says Nishimura. “The fact that FoxO and cholinergic neurons are also involved in insulin regulation in mammals suggests the mechanism revealed in this study has been conserved throughout evolution. We plan to further explore the elaborate survival strategies that organisms have developed to adapt to environmental changes.”
| Link to article | |
|---|---|
| Related links |