
News and Announcements from the CDB
Epithelial folding plays a crucial role in morphogenesis, turning two-dimensional (2D) epithelial sheets into three-dimensional (3D) structures. To initiate the folding process, some cells within the epithelial sheet need to undergo changes in cell shape. Cell shape changes often result from contraction of actomyosin network mediated by changes in localized apical myosin activity. However, there are some instances, such as dorsal fold formation in Drosophila embryos, in which folding occurs despite the lack of marked changes in myosin activity, suggesting that there are alternative ways to initiate epithelial folding that are myosin-independent.
In a study carried out by technical staff Michiko Takeda and Mustafa Sami, and Team Leader Yu-Chiun Wang of CDB’s Laboratory for Epithelial Morphogenesis, they use dorsal fold formation in early Drosophila embryo as a model to elucidate mechanisms underlying fold formation in the absence of localized myosin changes. They reported that a CAMSAP protein (microtubule minus-end binding protein), Patronin, plays a key role in reorganizing apical microtubule networks to change cell shape following a basal shift in polarity factors. Their findings were published in Nature Cell Biology.
Previous work led by Wang showed that prior to cell shape changes seen before dorsal fold formation, there is a change in cell polarity in the initiating cells as noted by a basal shift in proteins that regulate apical-basal polarity of the cells. In particular, the downregulation of Par-1, a MARK family kinase specifying basal-lateral membrane and involved in positioning cell-cell adhesive junctions to the subapical region of epithelial cells, resulted in the basal shift of junctions, and the shrinkage and descent of the cell apices, ultimately causing a reduction in cell height. However, it remained unclear how cell polarity changes affect cell shape.
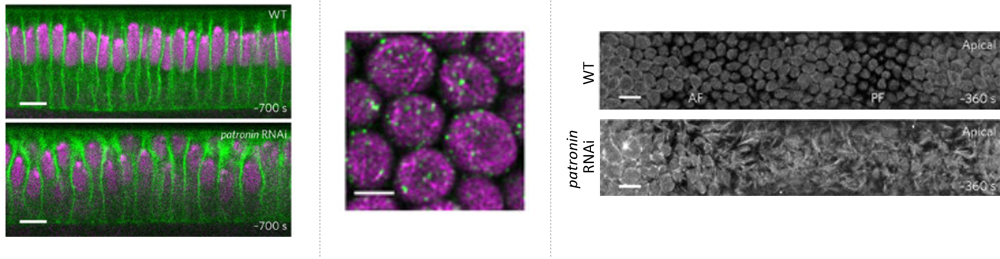
The team first turned their attention to a CAMSAP protein, Patronin, whose localization was recently reported to be regulated by Par-1, as a possible regulator of microtubule (MT) networks needed to modulate cell shape. The role of Patronin in epithelial cells was examined by generating RNAi-mediated patronin knockdown embryos, and loss of Patronin led to irregular apical surfaces, disparities in cell size and nuclear positions, whereas normally wildtype (WT) embryos have dome-like shape apices and show uniformity in cell size and nuclear positioning. Thus, these differences confirmed that Patronin plays a role in regulating cell shape. The team then performed live imaging of the apical surface of the dorsal epithelium and saw that in WT embryos, the apices initially appear rough prior to gastrulation, but gradually become smoother, and by gastrulation onset, have formed a spherically shaped membrane called the apical dome, and subapically, just below the dome, a ring-like microtubule structure. In contrast, patronin RNAi embryos exhibited fibrous and protrusive apices, and showed no dome or ring-like MT structure. They next tracked Patronin localization in relation to apical cellular dynamics and found that, as the embryo prepares for gastrulation, Patronin is seen localized in apical cortex in a punctate pattern. Super-resolution microscopy revealed that lining the inside of the WT apical dome was a filamentous network of unstable, non-centrosomal MTs, anchored by Patronin puncta to form a scaffold, and differed from the more basally localized centrosomal ‘inverted basket’ containing a mix of stable and unstable MTs. The team also found that the smoothening of the apical dome needs the motor protein Dynein that likely crosslinks the MT filaments to produce outward pushing forces. Together these observations indicate that Patronin regulates the apical MT network to ensure the formation of the apical dome.
The team next sought to determine whether Patronin and its associated MT network is involved in dorsal fold formation. They found that Patronin was redistributed more basally in the apical dome in the initiating cells where Par-1 is downregulated, and in par-1 RNAi embryos, Patronin displayed extensive basal distribution in all cells. To test whether the disparities in Patronin localization between initiating and neighboring cells are important for folding, the team overexpressed Patronin in the early embryo and found that saturating levels of Patronin across the tissue prevent folding. Together these results suggest that Par-1 acts to restrict Patronin localization in the apical region, and when downregulated, Patronin becomes basally redistributed to initiate folding.
As the apical MT network, made up primarily of unstable MT, likely undergoes rapid reorganization in response to polarity changes, the team searched for a possible regulator of MT networks and came across the MT-severing enzyme, Katanin, which has been reported to bind to Patronin. Katanin was found co-localizing partially with Patronin in the apical dome, whereas in patronin RNAi embryos, apical cortex localization of Katenin localization was reduced, suggesting that Patronin recruits Katanin to the apical cortex. Single-cell kymograph analyses of MT networks in WT, patronin RNAi, and Katenin depleted embryos revealed that Patronin is important for their architecture, while the concerted action of both Patronin and Katenin enhanced the remodeling, and furthermore, both Patronin and Katanin are needed to ensure uniformity of cell size, suggesting that network remodeling is crucial to maintain cell size homogeneity.
“Our study demonstrates that CAMSAP protein Patronin has a cell shape control function in epithelial cells that display minimal myosin changes. Before dorsal fold formation, the MT network is anchored apically by Patronin, and its remodeling ensures cell size homogeneity. At gastrulation onset, the network responds to basal polarity shifts to break the local force balance so that the epithelial sheet bends inward to fold,” explains Wang. “Mechanically, the apical dome of these early embryonic cells looks a lot like the canopy of an umbrella where the runner of the stretchers (Dynein) slide long the pole to exert outward pushing forces through the ribs (MT filaments) that are anchored to the canopy (membrane) at the rib tips (Patronin). Our next step will be to look into the counteracting forces that may be present at the inner surface of the canopy–or the apical membrane–to fully understand the force (im)balance states before and during folding.”
| Link to article |
|---|