
News and Announcements from the CDB
One of the fundamental questions in developmental biology is how an initially round fertilized egg develops into an elaborately shaped embryo. In Drosophila, whose eggs are elliptical in shape, the anterior-posterior (A-P) axis is specified by asymmetrical distribution of maternal determinants. In mammals such as humans and mice, the egg is spherical and the three body axes—A-P, dorsal-ventral (D-V) and left-right (L-R)—are established in order, which in turn determines future positioning of organs. The A-P axis, the first axis established in mammals, is determined by a group of cells in the extra-embryonic region called, distal visceral endoderm (DVE). How prospective DVE cells are selected in the embryo, however, has remained unclear as mammals do not appear to have A-P specifying maternal determinants as found in Drosophila.
In a study published in Nature Communications, visiting scientist Katsuyoshi Takaoka of the CDB’s Laboratory for Organismal Patterning (Hiroshi Hamada, Team Leader) report that, in mouse embryos, the DVE is randomly formed through a negative feedback by Lefty1 and Nodal on the cells that stochastically express the highest level of Nodal signaling during the blastocyst stage. The DVE then drives the establishment of the A-P axis by migrating to the future anterior side. Takaoka has since moved to the Max Planck Institute in Germany to continue his research.
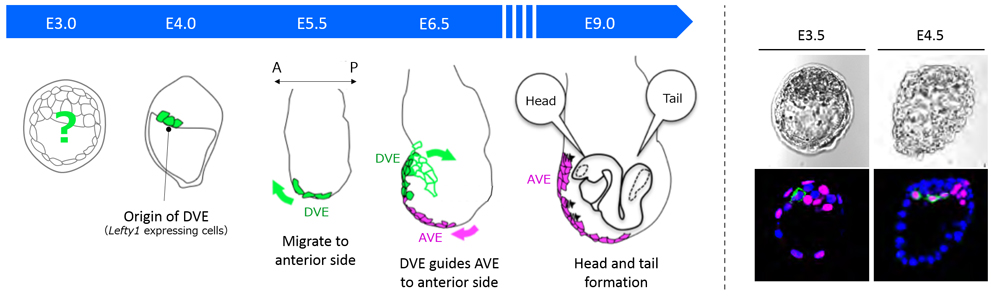
In the mouse, the DVE are selected from the primitive endoderm (PrE) at around embryonic day (E) 4, and then migrate to the future anterior end at E5.5. All visceral endoderm (VE) cells also undergo a shift at around the same time, resulting in localization of some VE cells at the embryo’s distal tip to become anterior visceral endoderm (AVE). The DVE then guides the AVE migration to the future anterior side for A-P axis establishment by E6.5, and eventually induces formation of the head region. In a previous study, Takaoka showed that Lefty1 expression is a marker for DVE progenitors, and succeeded in identifying prospective DVE cells as early as E4. But, they had not been able to pinpoint how the DVE progenitor cells, or Lefty1 expressing cells, were selected in the embryo.
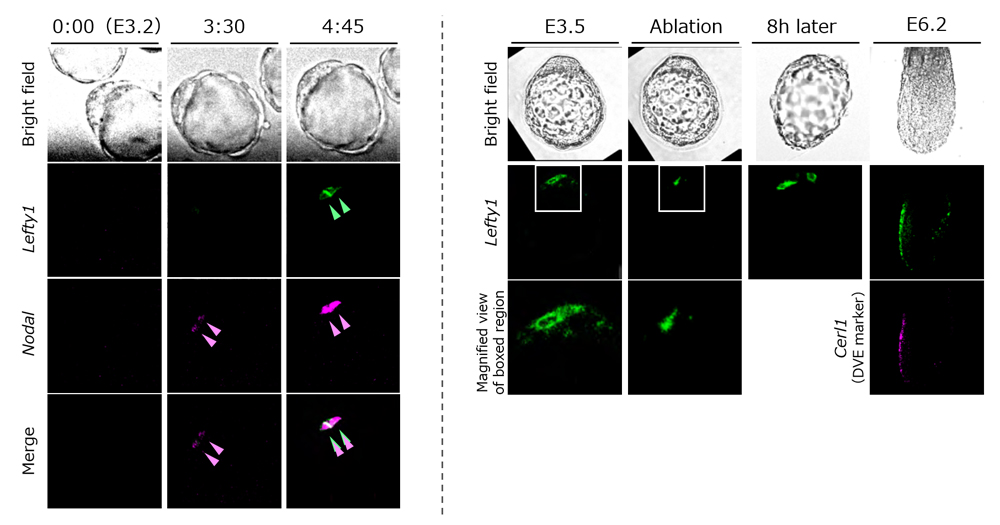
Lefty1 expression first appears in a subset of epiblast progenitor cells at around E3.5, and then in some cells of PrE at E4.5. Using mouse embryos at stages just prior to E3.5 and E4.5, the team first searched for a gene regulating Lefty1 expression, and identified transcription factor Foxh1, a transcription factor of Nodal signaling, as a direct regulator of Lefty1 expression. When Foxh1 or Nodal, the ligand for Nodal signaling, were inhibited in the embryos, Lefty1 expression was not detected, whereas endogenous Lefty1 was induced in an ectopic Nodal mRNA-injected cell or in a neighboring cell. They also examined Nodal and Lefty1 expression in the embryo using live imaging and discovered that while Nodal expression expanded rapidly from one cell to the surrounding cells, Lefty1 expression was restricted, being detected only in the first cell that expressed Nodal or the neighboring cells.
Why is Lefty1 expression seen only in a few cells? As Lefty1 is known to be a Nodal antagonist, Takaoka and his colleagues hypothesized that Lefty1 may serve as a negative regulator of Nodal signaling and decided to analyze the effects of inhibiting Lefty1 in embryos. In Lefty1 knockout embryos, they showed an enhancement of Nodal signaling and also the increasing number of prospective DVE cells. Thus, when the cell that initiates Nodal expression begins to express Lefty1, Lefty1 quickly diffuses to the other cells to inhibit Nodal signals, and also suppresses Lefty1 expression in the cells. Thus, this negative feedback mechanism appears to restrict the number of prospective DVE cells. When Lefty1 expressing cells were removed from blastocyts by laser ablation, another cell began to express Lefty1, and embryos subsequently continued to properly form DVE and eventually established the A-P axis. These results indicate that the cell showing high Nodal activity begins expressing Lefty1.
“All PrE cells have the potential to become DVE, and the DVE is formed from those cells that, by chance, experience the first strong Nodal signaling,” explains Takaoka. “Thus, the A-P axis is not yet determined at the blastocyst stage (E3), indicating that mammals have a different mechanism from the fly for establishing the body axes.”
Hamada adds, “We created many gene knockout embryos as well as manipulated the embryo by removing prospective DVE regions. However, all embryos were able to establish a proper A-P axis regardless of small differences seen in early DVE formation stages from wildtype embryos. Our results indicate that the molecular mechanism underpinning A-P axis determination is flexible yet robust. As there is still much we do not know about the early developmental mechanisms and origins of symmetry breaking in our bodies, we are keen to continue searching for the answers.”
| Link to article |
|---|