
News and Announcements from the CDB
Vertebrates have three main body axes that are determined in turn during early stages of embryonic development: anterior-posterior (A-P) axis, dorsal-ventral (D-V) axis, and finally the left-right (L-R) axis. L-R axis determination is mediated by events in a region of the early embryo called the node, a transiently formed shallow depression at the ventral region of the embryo consisting of approximately 200 to 300 cells. Each node cell possesses a single cilium that moves in a clockwise rotation, which together creates unidirectional fluid flow (nodal flow) in a leftward direction leading to the breaking of left-right symmetry, in other words create the L-R axis, by inducing expression of a left-determining factor, Lefty, on the future left side of the body. There are, however, many gaps that remain in our understanding of how A-P and D-V positional information is detected by the node cells to correctly establish the L-R axis.
A new study by research scientist Katsura Minegishi in the Laboratory for Organismal Patterning (Hiroshi Hamada, Team Leader) and other collaborators has analyzed the molecular mechanisms involved in generating unidirectional nodal flow leading to L-R symmetry breaking using mouse embryos. Their meticulous work, published in Developmental Cell, revealed that a Wnt5 expression gradient along the A-P axis of the embryo induces polarization of planar cell polarity (PCP) proteins in the node cells which causes the basal body of their cilium to shift to a more posterior position. This shift in basal body position tilts the angle of the rotational axis toward the posterior direction, consequently creating the leftward nodal flow that is critical for breaking L-R symmetry.
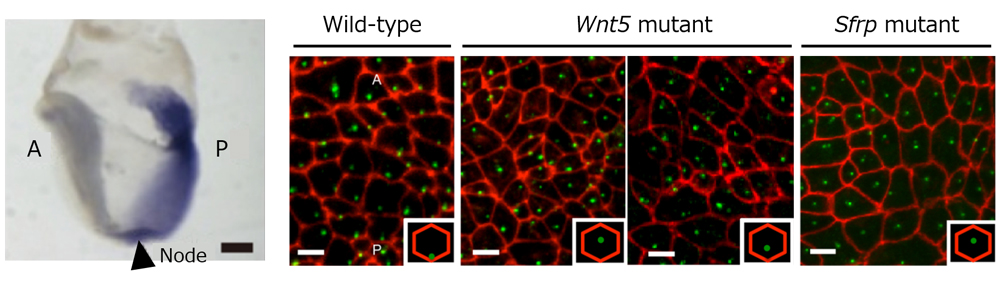
In the mouse, the node appears transiently in the embryo around embryonic day 7.5. Initially, the basal body of the cilium is found at a central region of each node cell and rotates in a clockwise direction to create small swirling fluid flows. As development progresses, the basal body position gradually shifts posteriorly, causing the rotational axis of the cilium to tilt in a posterior direction which collectively creates a strong leftward flow. The laboratory previously reported that this unidirectional nodal flow is critical for determining the region where Lefty expression is induced, thus breaking L-R symmetry. Furthermore, node cells in the node exhibit polarity along the A-P axis through asymmetrical localization of planar cell polarity (PCP) proteins mediated by planar cell polarity (PCP) mechanisms that control intercellular polarity across a plane of cells. This also appears to contribute to the posterior shifting of basal body needed to create leftward flow, but the signals involved in positioning of basal body remained unclear.
The group first turned their attention to Wnt5 proteins as potential candidates involved in basal body positioning, as Wnt signals are often found upstream of the PCP pathway, and Wnt5 is known to be expressed in a gradient along the A-P axis of the embryo near the node, with lower concentrations on the apical side and higher concentrations on the posterior side. To determine whether Wnt5 plays a role in basal body positioning in node cells, they examined Wnt5 knockout (KO) embryos and found that the basal body of many node cells failed to shift posteriorly, consequently disrupting unidirectional leftward nodal flow. They surmised that another factor was also involved in establishing the Wnt gradient as posterior shifting of basal body was still seen in some node cells of Wnt5 KO embryos. They examined patterns of Sfrp, a known Wnt antagonist, as a possible candidate and discovered that Sfrp KO embryos showed similar phenotypes as Wnt5 KO embryos, if not a more pronounced effect, with respect to failure of basal body shifting. Posterior basal body shift was also not observed in a series of experiments in which Wnt5 or Sfrp gradients were disrupted by ectopic or over-expression of either gene in the node, indicating that both Wnt5 and Sfrp gradients are essential for shifting the basal body position posteriorly.
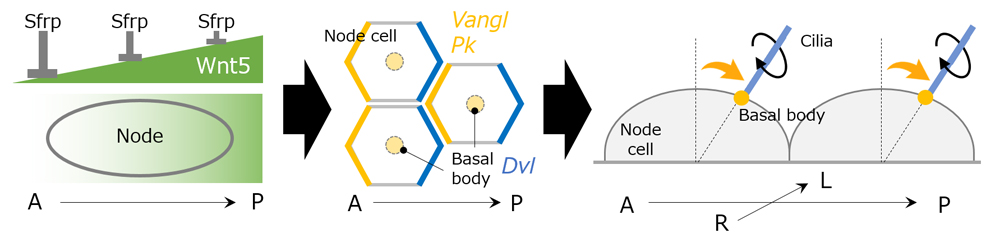
The group next examined how the node cells detect the Wnt5 gradient established within the node, focusing on intracellular localization of PCP proteins within the node cells. They confirmed that anterior localizing protein Vangl, and posterior localizing protein Disheveled (Dvl) were expressed at respective poles of node cells following establishment of the Wnt5 gradient. They also revealed that another PCP protein, Prickle (Pk), was expressed at the anterior end of node cells. Pk KO experiments indicated that Pk was required for anterior localization of Vangl as well as to ensure proper shift in basal body position to create leftward flow. PCP proteins were also found to interact with and influence neighboring node cells, as when Pk localization in one node cell was disrupted, abnormal basal body positioning was seen in surrounding cells. Thus, both intracellular and intercellular communication of PCP proteins appears to be involved in detecting the Wnt5 gradient within the node and the node cells themselves.
“While we have shown in this study that Wnt signaling is the initial cue triggering the posterior shift of the basal body, it is still not clear how individual node cells can sense small differences in levels of intracellular Wnt activity at anterior and posterior ends. Nor do we understand how the basal body shifts to a more posterior position,” says Hamada. “We plan to continue exploring these unanswered questions in order to fully understand the mechanisms of L-R symmetry breaking.”
| Link to article |
|---|