
News and Announcements from the CDB
Tubular networks in the body are essential for transporting fluids such as blood through blood vessels, or promoting exchange of gases in the trachea and bronchiolar structures of the respiratory system. Formation of tubular networks are triggered by signaling factors secreted during early stages of development, which directs some cells to become so-called ‘leading’ tip cells to begin protruding outward from an initially spherical epithelial cyst and lead ‘follower’ stalk cells to specific locations to form the branching structures. In tracheal tube formation, FGF signaling is known as the major branching signal for specifying tip cells. Whereas in most animal species only one tip cell is specified, in Drosophila, two tip cells are in fact specified by FGF. Interestingly, these two tip cells will later differentiate into two distinct cell types. One becomes a fusion cell (FC) that connects branches of tracheal primordia from adjacent body segments together by undergoing topological change into a donut shape (see Science news: May 2, 2016), and the other differentiates into a terminal cell (TC), which acquires a complex bifurcating and lumenal structure, and functions similar to bronchioles. The mechanism underlying the divergent cell fates of tip cells remained poorly understood.
Now, a study led by research scientist Guangxia Miao of the Laboratory for Morphogenetic Signaling (Shigeo Hayashi, Team Leader) zooms in on the Drosophila tracheal primordia and the mechanisms involved in sequential specification of two tip cell fates by a single inductive FGF signal. Their work, published in Development, revealed that whereas FGF initially specifies FC fate and directs branch migration to their destination, once there, the expression of transcription factor Escargot (Esg) suppresses FGF signaling in the FC and permits activation of FGF signaling in an adjacent cell to differentiate into a terminal cell (TC), resulting in two cells with distinct properties.
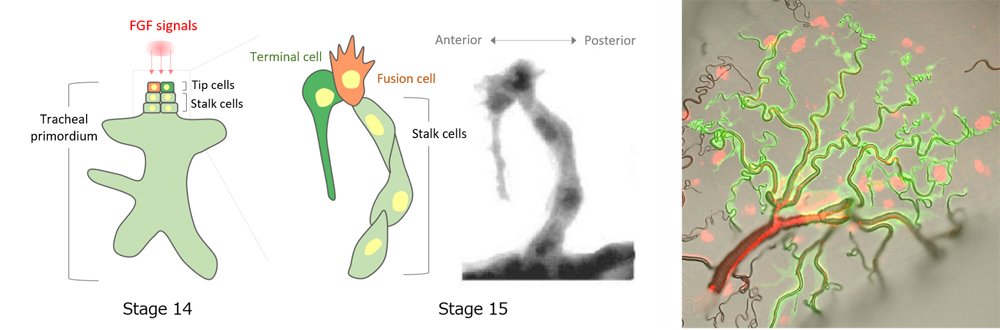
FGF signaling has been known to play multiple roles during tracheal tube formation. First, it specifies the FC-type of tip cells that will lead branch migration. Following specification, FCs will in turn inhibit FGF signaling in neighboring cells through activation of Notch-Delta, converting them into stalk cells that will trail behind tip cells. FGF signaling is also involved in guiding FCs migration and differentiation of tip cells into TCs. How this single FGF signal can control multiple aspects of cell differentiation and cell motility was unclear.
The team began by analyzing the distribution of the cells in which FGF signaling was activated. During early stages of tracheal development, downstream factors of FGF signaling were activated in a small group of cells in the primordium that includes the future FC and TC, but by stages 13 to 15, FGF signaling in these cells, with the exception of the TC, was lost. The team then turned their attention to Esg, whose expression appeared to be linked with FC specification. Analysis of Esg mutant flies revealed that FCs in these mutants failed to fuse with its partner FC, continued to migrate and elongate, and as seen in TCs, formed bifurcations. Further, Esg-mutant FCs were confirmed to show TC-like properties such as activated FGF signaling and expression of TC-specific marker genes. These findings indicated that Esg is expressed specifically in FCs, functioning to suppress FGF signaling and prevent FCs from differentiating into TCs.
When the team used live-imaging to track FC and TC positions, they found that between stages 14 to 15, FCs and TCs always undergo an anterior-to-posterior positional shift. This positional shift appeared to be critical for establishing the tracheal network, but it was not clear what signal was controlling this event. Using the technique previously developed by the lab, in which ectopic FGF expression could be induced at any place and time by laser irradiation, they examined the behavior of FCs and TCs to FGF expressed ectopically in different areas around the nascent tube. FCs moved away from FGF signals, whereas TCs moved toward FGF signals and created new bifurcations. In Esg mutants, FCs lost its repulsive response to ectopic FGF. Thus, the positional shift is brought about by the difference in responses between FCs and TCs to FGF signals.
“Analogous to train track switching devices, Esg coordinates time-lagged cell responses of tip cells to FGF, which results in two cells with distinct properties,” explains Hayashi. “There are more cell types in the body than there are signaling factors. Thus the cells must adopt strategies allowing it to reuse a single signal to generate cell type diversity, and this study gives a glimpse into one such mechanism that evolved in organisms.”
| Link to article | |
|---|---|
| Related links |