
News and Announcements from the CDB
Epithelial cells are arranged as polarized monolayer sheet and are often found lining the surface or lumen of organs. The epithelial cell edge that comes into contact with fluids or the external environment is referred to as the apical side, while the opposite side that attaches to connective tissues is referred to as the basal side. To maintain the sheet-like configuration, epithelial cells attach to each other along their lateral membranes through a number of special junctional structures. Near the apical side is a complex of ‘tight junction’ and ‘adherens junction,’ known as the apical junctional complex (AJC), which is lined with a bundle of actin filaments (F-actin) lies beneath the apical membrane forming a circumferential actin belt, and has been well studied to date. Below the AJC is a junctional structure called lateral membrane contacts (LCs), which spans most of the lateral membrane region and whose functions are not as well documented.
Now, a study led by visiting researcher Tamako Nishimura in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Team Leader), published in the Journal of Cell Biology, took a close look at LCs of epithelial cells and uncovered a role played by DAAM1, a formin family protein associated with actin polymerization, in stabilizing the adhesion of lateral membranes of adjacent epithelial cells. They also revealed the molecular mechanism underpinning instability of LCs in the absence of DAAM1.
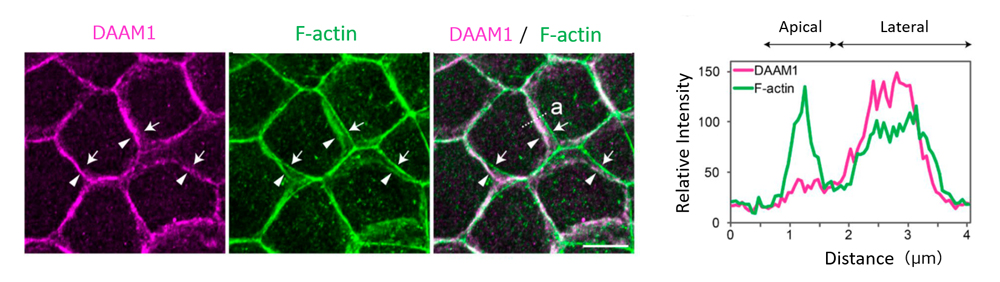
Left: DAAM1 was localized at LCs of epithelial cells. When cells viewed from a direction perpendicular to the cell layer, DAAM1 localization was seen to overlap with F-actin distributed in lateral membrane (arrowheads) as opposed to F-actin localized in the apical actin belt (arrows). Right: Tracings of relative signal intensity of DAAM1 and F-actin distributed along dotted line ‘a’ in the image to the left.
In a past study from the same laboratory also led by Nishimura, in which they reported the mechanism involved in apical constriction of neural epithelial cells during neural tube formation, DAAM1 was identified as one factor recruited to the cell-cell adhesion sites to mediate constriction (see Science News: May 28, 2012*). Studies by other groups have reported observations of abnormal sarcomere formation and cell-cell adhesion between cardiomyocytes in DAAM1 mutant mouse, and of reduced DAAM1 expression possibly contributing to invasiveness of astrocytoma cells, one type of brain tumor cells; thus, suggesting DAAM1 playing another role in regulation of cell-cell adhesion (junctions).
Nishimura’s team used a mouse mammary gland epithelial cell line to first examine the localization of DAAM1, and found that it accumulated at epithelial junctions along the lateral membrane. F-actin was also distributed along the lateral membrane, overlapping with that of DAAM1, and differing from F-actin distribution seen in the apical actin belt. When DAAM1 was knocked down, they observed reduced F-actin localization along the lateral membrane, as well as larger tilt angles of LC membranes. Time-lapse imaging revealed more dynamic lateral membrane movements in DAAM1-depleted cells compared to control cells. When cells were cultured in a gel matrix for several days, control cells formed spherical cysts, with cells arranged in radial manner, whereas DAAM1-depleted cells formed disorganized aggregates, with some cells appearing to be extruded out from the aggregates. Interestingly, in co-culture of control and DAAM1-depleted cells, DAAM1-depleted cells extended long protrusions into nearby cell layers, reminiscent of behavior seen in invasive cells, when flanked by control cells. Hence, these results suggest that DAAM1 mediates stability of cell junctions through F-actin, which in turn contributes to maintenance of epithelial integrity.
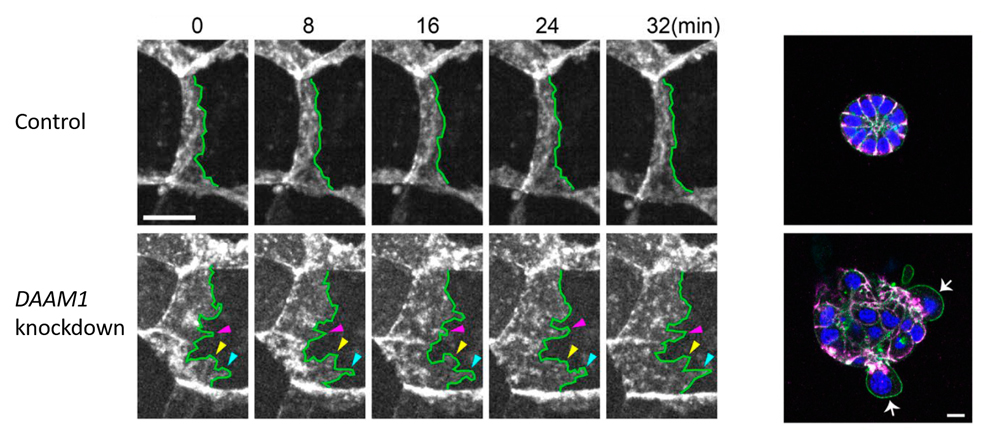
Left: DAAM1 knockdown results in abnormal tilting of lateral membrane faces, and exhibit more dynamic motility of the lateral membrane than control cells. Right: When cells were cultured in Matrigel for several days, control cells formed cysts in which cells were arranged radially, whereas DAAM1-depleted cells showed disorganized aggregation with some cells being extruded from the cell mass.
The team next investigated why the LCs become unstable after DAAM1 depletion. During normal cell migration, cell edges in the direction of migration move dynamically forming lamellipodium, and its motility is known to be mediated by a molecular complex called the WAVE complex. The team thus speculated that the WAVE complex also plays a role in LC instability following DAAM1 depletion. They analyzed the WAVE complex components in DAAM1-depleted cells and identified WAVE2 and its upstream regulator Rac as effectors of LC instability, resulting in acquired membrane motility.
“Our current study demonstrated that DAAM1 maintains stability of epithelial cell junctions by suppressing WAVE complex-mediated membrane mobility,” explains Nishimura. “It was surprising to discover that epithelial cells, which are considered stable when packed together, in fact harbor the potential to acquire motility.”
Takeichi adds, “The WAVE complex–mediated motility of LC membranes is likely critical for repair mechanisms involving cell migration, such as wound healing. At the same time, errors in DAAM1 regulation of the WAVE complex may lead to cancer cell-like invasive behavior. Therefore, detailed studies on DAAM1 functions in vivo may reveal clues into mechanisms of cancer invasion.”
| Link to article | |
|---|---|
| Related link |