
News and Announcements from the CDB
The approach of winter signals the start of the hibernation period for some animals, such as squirrels and bears. Hibernation, a period of days or weeks of inactivity, can be considered an extreme form of energy conservation as some animals are known to lower their metabolic rates to just a few percent of normal awake levels, thereby reducing demand for oxygen during this period. A shorter hibernation-like state (minutes to hours) called torpor is also induced in some animals, and is marked similarly by active reduction in metabolism (hypometabolism). The underlying mechanisms regulating hibernation and torpor remain largely unknown as hibernating and torpor species are few and far between, making them difficult subjects to use in research. Recently, however, there have been reports that mice, which are commonly used in research, can undergo torpor under certain conditions, hinting at possibilities of studying this phenomena in the laboratory. Understanding how active hypometabolism can be induced may benefit humans as well in clinical applications, where reducing oxygen consumption by inducing hypometabolism could slow down progress of brain damage after stroke, or help prolong storage periods for organs or tissues for transplantation.
In a new study led by research scientist Genshiro Sunagawa in the Laboratory for Retinal Regeneration (Masayo Takahashi, Project Leader), published in the online journal Scientific Reports, they created an algorithm for detecting torpor based on actual measured values of baseline metabolism of individual mice, which can be used to stably observe and evaluate torpor states of mice. The team further analyzed the mouse thermoregulatory system between normal (awake) and torpor states to reveal a common feature between torpor and hibernation.
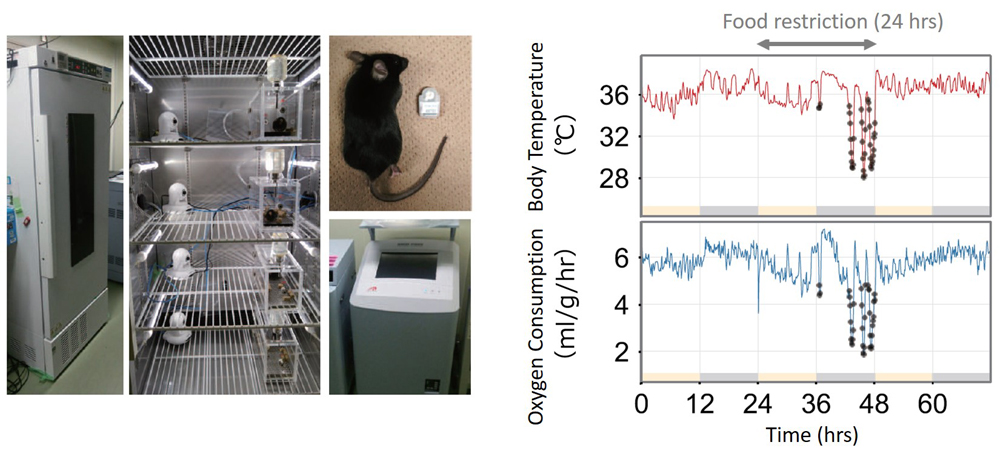
Warm-blooded animals have an internal thermoregulatory system to maintain a relatively stable body temperature. When temperatures in the outside environment drop, the body is more prone to losing heat, and consequently begins to actively generate heat to compensate for the heat loss. Normally, if this mechanism stops functioning and the body temperature falls below a certain threshold or reference temperature, the tissues and cells incur damages and eventually die, but this does not occur in hibernating or torpid animals. Instead, these animals appear to possess a mechanism that signals to the body to abandon or suppress its normally active thermoregulatory system.
Torpor takes place over a shorter period and is more unstable compared with hibernation, and even shows great variation between individual animals. This has made it difficult to clearly define and evaluate states of torpor. The team prepared a chamber fitted with sensors to measure and record body temperature and oxygen consumption of mice under controlled ambient temperature conditions. Baseline metabolism of the individual mice were calculated from the data collected over 24 hours of observation during which time they had no physical contact with researchers, and values falling outside the range of the baseline metabolism (outliers) were defined as ‘torpor.’ Using their model to evaluate torpor states, they found that a constant ambient temperature between 12°C to 24°C and restricting access to food for 24 hours could stably and reliably induce torpor in mice.
The team then created a mathematical model for body’s thermoregulatory system in response to ambient temperature changes, and using the data collected from the above experiments, estimated the parameters that changed significantly. Parameters included how easily the body loses heat, the reference temperature the body is trying to maintain, the actual body temperature, and the sensitivity of the thermoregulatory system. During hibernation, both the reference temperature and sensitivity of the thermoregulatory system show a dramatic reduction. In torpid mice, they find that reference temperature shows only minimal changes, while the sensitivity of the thermoregulatory system falls to levels similar to that seen in hibernation.
“Our study demonstrates that a common feature of hibernation and torpor is that sensitivity of the thermoregulatory system is significantly reduced during these states. This suggests that to induce active hypometabolism there is either a signal relayed to the body to suppress or reduce thermogenesis, or there is a mechanism signaling to the body to ignore the default thermogenesis system. Our goal is to unveil the complete mechanism regulating this phenomena,” explains Sunagawa. “This work also paves the way to use mice as a model to understand active hypometabolism, as well as seek possibilities for applying hypometabolism in clinical applications.”
| Link to article |
|---|