
News and Announcements from the CDB
The hippocampus is the seat of memory formation and learning, and disturbances of hippocampal function are known to lead to cognitive and psychiatric disorders, making this a brain region of fundamental importance in a range of biomedical research fields. In developmental terms, the hippocampus emerges through the activity of an organizing center on neighboring tissues, but its location deep in the embryonic brain makes it difficult to study experimentally, and the mechanisms by which hippocampal cells are induced remain unclear.
Now, junior research associate Hideya Sakaguchi and others from the Laboratory for In Vitro Histogenesis (Mototsugu Eiraku, Team Leader) and the former Laboratory for Organogenesis and Neurogenesis (Yoshiki Sasai, dec.) report the induction of self-organizing hippocampus-like tissue from human embryonic stem cells (hESCs). Published in Nature Communications, this work provides a new experimental resource for studies ranging from hippocampal development to Alzheimer’s disease.
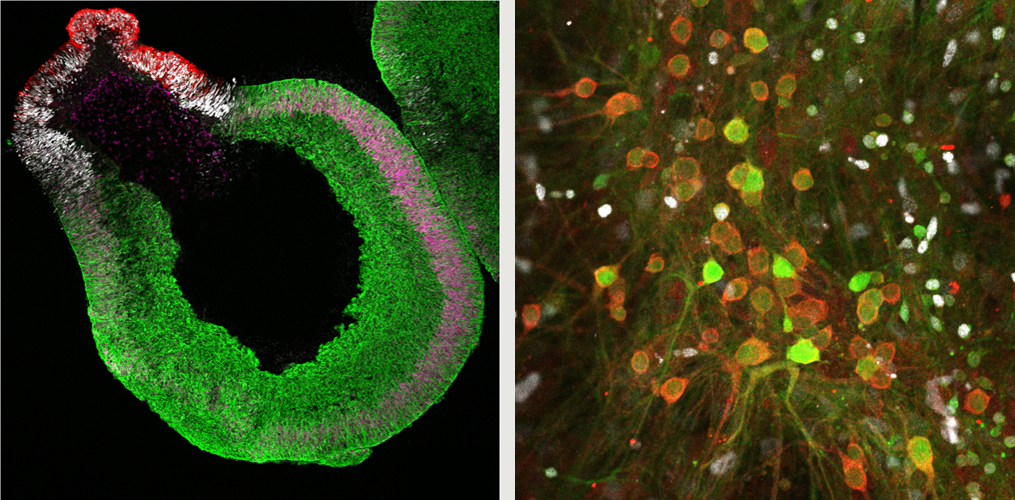
Top left: Neuroepithelium at day 42 in vitro. Cortical hem (negative for TTR and Lmx1a) and medial pallium
(green, Foxg1::Venus; magenta, Lef1) form sequentially adjacent to the choroid plexus (red, TTR; white, Lmx1a).
Top right: At day 179 of culture, granule (Prox1, white) and pyramidal (KA1, red) neurons are seen (middle).
Bottom: Calcium imaging shows synchronized depolarization at day 143.
This new report represents the most recent in a series of experiments aimed at inducing neuronal differentiation from pluripotent stem cells stretching back more than a decade, and makes use of the SFEBq (serum-free floating culture of embryoid body-like aggregates with quick reaggregation) method developed by the same group. In this study, the group sought to adjust SFEBq conditions to steer hESCs toward the primordial tissue in the embryonic brain (called medial pallium) from which the hippocampus arises.
Using a previously reported SFEBq technique for inducing self-organized cerebral cortex-like tissue, Sakaguchi sought to develop a cocktail of factors delivered at the right times and dosages to trigger hippocampal differentiation from hESCs. By tweaking the levels of Wnt and BMP4, dorsalizing factors that strongly influence neuronal differentiation, the group was able to induce the expression of genetic markers of choroidal plexus, the neighboring cortical hem, and finally medial pallium, suggesting that a self-organized form of differentiation was occurring in the same chronological order seen in normal development.
They next attempted to generate hippocampus-like tissue from the self-organized medial pallium. By experimenting with different culture media and conditions, they gradually discovered a reliable method for directing the medial pallium into hippocampus, as evidenced by marker gene expression. As these cell colonies begin to destabilize by about 100 days in vitro, they dissociated them into individual neuronal cells between 73 and 84 days of culture and followed their developmental course. After isolation, these individual cells re-aggregated, and by day 197 around 75% expressed markers, indicating hippocampal differentiation. Moreover, markers of two distinct neuronal cell types—pyramidal and granular—were each observed in about one-third of the total population, and glial- and astrocyte-like cells were present as well.
Tests of neuronal function by patch-clamping confirmed that the differentiated neuronal cells possessed working synapses, and calcium imaging at day 143 showed synchronized depolarization, suggesting that the hippocampal tissue was exhibiting network-like activity.
“The ability to recapitulate hippocampal development in vitro is a significant step forward,” says Eiraku. “We look forward to the use of this system not only to study biological phenomena such as patterning and regionalization, but as a resource in biomedical research into diseases like Alzheimer’s and schizophrenia as well.”
| Link to article | |
|---|---|
| Related link |