
News and Announcements from the CDB
Retinitis pigmentosa (RP) is a degenerative condition that ultimately damages the photoreceptors at the back of the eye, one of the many layers of specialized cells in the complex retina. Patients afflicted with RP suffer from loss of visual acuity, poor night vision and a narrowed visual field. Despite ongoing efforts to develop effective treatments using gene transfer and bioengineering approaches, to date RP remains resistant to therapy. The emerging paradigm of cell therapy represents another potential alternative, and the 4D cell culture techniques developed by CDB Team Leader Mototsugu Eiraku and former Group Director Yoshiki Sasai (dec.), in which embryonic stem cells (ESCs) can be steered to give rise to highly organized, three-dimensional tissue-like structures in vitro provides a promising starting point for the development of potential cellular resources for transplantation.
Now, a new study by Hiroshi Shirai, Michiko Mandai and others in the Laboratory for Retinal Regeneration (Masayo Takahashi, Project Leader) reports the successful transplantation of human ESC-derived retinal tissue into a non-human primate model of retinitis pigmentosa. This work, published in the Proceedings of the National Academy of Sciences, represents an exciting new step toward the development of cell-based treatments for RP.
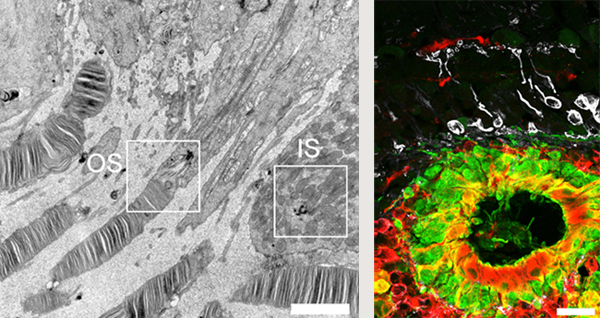
Transplantation of hESC-derived retinal precursor tissue into immunocompromised rat yields mature photoreceptor cells with both inner
and outer segments (left). Transplantation of hESC-derived retinal precursor tissue into subretinal space in a primate model of RP
produces mature photoreceptors (green) proximate to host bipolar cells (white) suggestive of synapse formation (right).
The same group had previously shown that retinal tissue generated from mouse ESCs and induced pluripotent stem cells (iPSCs) is capable of maturation and integration into host tissue in mouse models, which prompted to them to move one step closer toward clinical testing by attempting a similar approach using macaque models of RP, which have the advantage of closer similarity to humans.
Before attempting transplants in macaques, the group first used a rat model to identify culture conditions for generating retina-like tissue with the ability to survive, mature, and function in the host environment. After experimenting with a range of differentiation times and culture conditions, they determined that slightly less mature cells, at 50–60 days of in vitro differentiation, produced superior grafts in terms of thickness and ability to integrate.
Next, the group needed to develop a monkey model of RP, a disease in which degeneration progresses from the peripheral part of the eye inwards, resulting in visual acuity losses in the center of the field of vision. The scientists used two different approaches to simulate this radiating degeneration, chemical injections and photocoagulation by laser light, producing selective foci of damage suitable for use in transplantation experiments and subsequent monitoring.
They next transplanted hESC-derived retina tissue after ~60 days of differentiation into the subretinal space of immune suppressed macaque RP models. They observed proliferative growth of the grafted tissue for up to 120 days, and ongoing differentiation as evidenced by the expression of early photoreceptor markers by day 90 and markers of rod and cone cells by day 150 of differentiation. Additionally, they observed growth patterns suggesting the formation of synaptic connections between host bipolar cells and graft photoreceptors, although at low frequencies.
In establishing primate models for preclinical studies of cell transplantation in retinitis pigmentosa, this study opens up new possibilities for testing of different culture and surgical delivery techniques. “In the future, we hope to test whether iPSC-derived tissue will show similar ability to integrate and function in the host retina,” says Mandai.
| Link to article |
|---|