
News and Announcements from the CDB
Embryonic stem cells (ESCs) are perhaps the most recognized type of pluripotent stem cell derived from mammalian embryos, but they have a lesser known cousin as well, called epiblast stem cells (EpiSCs). Whereas mouse ESCs are obtained from the inner cell mass (ICM) at the blastocyst stage of development, and can give rise to all three germ layers as well as reproductive germ cells, their epiblast counterparts are derived from a later stage, after the embryo has implanted into the uterine lining, and no longer have the capacity for germ cell differentiation. In recent years, these variant states of pluripotency have come to be described as naïve and primed, respectively, and the transition to a primed state in mouse is now known to be controlled by the activin and FGF pathways. Human ESCs in contrast are already in a primed state, and efforts to obtain naïve ESCs from the ICM have not borne fruit, highlighting interspecies differences in pluripotency.
Now, in a report published in e-Life, a joint study by the Laboratories for Sensory Development (Raj Ladher) and Early Embryogenesis (Guojun Sheng) has identified naïve pluripotency in cells obtained from zebra finch eggs immediately after laying. This lays the groundwork for expanded studies into the establishment and maintenance of pluripotency across amniote species, not only mammals. Ladher and Sheng have respectively since moved to the National Centre for Biological Sciences (Bangalore) and Kumamoto University.
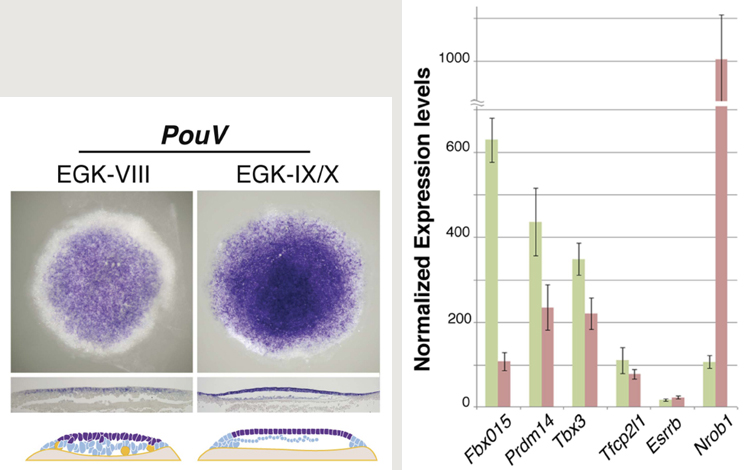
Left: Expression of PouV in stage 8–10 zebra finch egg. Right: Expression levels of naïve pluripotency markers in cells from zebra finch (green) and chicken (red) eggs.
Chicken and quail are the most commonly used avian species in developmental research, but their embryos have already developed substantially by the time the egg is oviposited. In this study, however, the Ladher and Sheng labs recognized that the comparatively less advanced development of zebra finch embryos at the time of oviposition afforded an opportunity to look for naïve pluripotent stem cells in bird.
The team first observed embryos at this stage and determined that they represented a state of development (stages 6–8) roughly comparable to the mouse blastocyst prior to epithelialization. Chicken embryos from laid eggs in contrast have already undergone this transition and correspond more closely to mouse epiblast.
Looking at the molecular profiles of zebra finch embryos confirmed what their morphology suggested; not only did they express canonical markers of pluripotency such as Nanog, PouV and DNMT3b, but numerous markers of naïve pluripotency as well. The expression of the latter markers dropped precipitously after stage 10, consistent with a clearly timed transition. Importantly, stage 6–8 embryos also expressed germline markers, which lines up well with another feature of naïve pluripotency in mouse ESCs. Interestingly, markers of naïve pluripotency were also observed, although at generally lower levels, in cells from chicken embryos at a later stage (~10) of development.
These findings point to the prospect that naïve and primed pluripotency may be conserved across amniote taxa, not only in mammals. “We’re hoping to one day identify bona fide pluripotent stem cells from zebra finch embryos, which would enable the study of mechanisms behind the establishment and maintenance of pluripotency in a much wider range of model systems,” says Ladher.
This new work involved not only performing cell and molecular biology experiments in zebra finch embryos, but establishing the bases for rearing and housing zebra finches under laboratory conditions. The same joint team published their methods for zebra finch breeding in the October issue of Genesis, in the interests of adding new experimental options for avian embryological research.
| Link to article | |
|---|---|