
News and Announcements from the CDB
The brain’s cerebellar cortex, which is associated with motor control, consists of multiple cell types arranged in a layered structure; from top to bottom, the molecular layer, the Purkinje cell layer and the granule cell layer. The Purkinje cells, found in the thin Purkinje cell layer, have a large cell body with highly arborized dendrites fanning out toward the molecular layer above and a long axon extending down to the deep cerebellar nuclei; they play a central role in processing information received from outside the cerebellum. The granule cells in the granule cell layer are much smaller and play a role in transmitting the information received from throughout the body to the Purkinje cells. These two cell types arise from the two distinct regions of the developing cerebellum and later mesh together to form the layered structure of the cerebellum. While there are reports of the successful induction of certain cerebellar neurons in vitro, recapitulating the developmental processes to generate three-dimensional (3D) cerebellar structure has proven to be a challenge.
Now, new work led by research specialist Keiko Muguruma of the Laboratory for Organogenesis and Neurogenesis (Masatoshi Takeichi, Team Leader), former group director Yoshiki Sasai, and other colleagues details a method to generate functional cerebellar neurons from human embryonic stem cells (hESCs) in vitro. They also show that these induced neurons can self-organize to form a 3D structure closely resembling the human cerebellar cortex seen in the early stages of development. Published in Cell Reports, their work demonstrates how the addition of essential growth factors at key time points can produce cerebellum-like structure in a culture system.
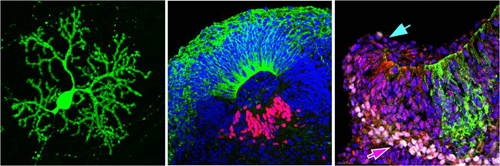
The laboratory focuses on recapitulating early embryonic developmental processes in vitro to form 3D tissue structures, using the principle of self-organization. In a previous study also led by Muguruma, they successfully generated cerebellar neurons from mouse ESCs (mESCs) at relatively high efficiencies (see CDB news: September 18, 2010). There, using the SFEBq (serum-free culture of embryoid body-like aggregate) method also developed by the laboratory, they induced the formation of Purkinje neurons by adding FGF2 and insulin to the medium. The further addition of bone morphogenetic protein 4 (BMP4) to the system produced granule cells; however, they were not able to induce the generation of both Purkinje neurons and granule cells simultaneously. Muguruma et al. took this as the starting point for the current study and attempted to apply their method to produce cerebellar neurons from hESCs.
The initial attempts to induce cerebellar neurons from hESCs with the mouse ESC method were met with difficulties; regardless of whether or not a ROCK inhibitor which promotes cell survival and reaggregation of hESCs was added to the 3D culture system, the resulting floating aggregates were extremely fragile, making it difficult to maintain long-term cultures. This problem was solved by adding transforming growth factor β (TGF-β)-receptor blocker, a known promotor of neuroectoderm differentiation, to the system. As with mESCs, Muguruma et al. then confirmed that adding fibroblast growth factor 2 (FGF2) leads to the induction of isthmus organizer-like tissue in the hESC aggregates within 14 days in culture, and that by day 35, these aggregates also formed hollowed rosette structures within. These hollowed rosettes also expressed markers in a pattern typically seen in cerebellar plate neural epithelium (CPNE), which later gives rise to cerebellum-specific neurons.
The group next examined whether these CPNE cells have the potential to generate mature cerebellar neurons. As synaptogenesis between granule cells and Purkinje cells promotes maturation of Purkinje cells, the FGF2-treated aggregates were dissociated and cocultured with granule cells isolated from mouse tissues. They found that cells that expressed an early differentiation marker for Purkinje cell progenitors differentiated into mature Purkinje cells, identified by their characteristic morphology and expression of late Purkinje-cell markers. Electrophysiological experiments on these hESC-derived Purkinje cells revealed that they also show functions similar to naturally born Purkinje cells.
A closer look at the hollowed rosettes formed in the aggregates at day 35 revealed that while many were small and round, there were a few that were relatively large and elliptical in shape. The elliptical structures expressed dorsal-specific markers on the outside and ventral-specific markers on the inside, which resembles the dorsal-ventral (D-V) polarity seen in the neural tube. Muguruma et al. tested a range of conditions and found that adding FGF19 from day 14 promoted the formation of these elliptical structures. The further addition of SDF1, which has been linked to cerebellar development, at day 28 led to the formation of a large continuous neuroepithelial structure resembling the CPNE, rather than the formation of multiple elliptical structures inside the aggregate. SDF1 also promoted differentiation of the continuous neuroepithelium to generate a three-layered structure—the ventricular zone, Purkinje cell precursor zone and rhombic lip-derived zone—similar to normal cerebellar development. They noticed that the periphery of one end of the hESC-derived neuroepithelium was curled, and exhibited gene expression patterns seen in the rhombic lip which normally generates the granule cells in vivo. Thus, cerebellar development, at least the early stages, can be recapitulated in a 3D hESC culture system with sequential addition of key factors, FGF2, FGF19, and SDF1.
Muguruma and her colleagues are also attempting to establish cerebellar disease models from patient-derived hiPS cells by adapting their current protocol for human iPS cells. These attempts to generate cerebellar tissue in vitro could lead to understanding disease pathology or to applications in drug screening.
“Cerebellar development involves a very dynamic change in tissue morphology. So far we have only been able to generate embryonic cerebellum, roughly equivalent to the first trimester,” says Muguruma. “But, if we can produce a more mature cerebellum tissue in vitro, it may reveal the mechanisms underlying the dramatic morphological changes taking place during development.”
| Link to article |
Self-Organization of Polarized Cerebellar Tissue in 3D Culture of Human Pluripotent Stem Cells |
|---|---|
| Related link |
Purkinje neurons from ES cells(Sep 18, 2010) |