
News and Announcements from the CDB
Retinitis pigmentosa (RP) is a group of genetic diseases of the eye that are characterized by the degeneration of photoreceptors, a type of neural cell which detects light. Symptoms of RP include night blindness and progressive narrowing of peripheral vision, and in some advanced cases, may lead to blindness. The pathogenesis of this disease is as varied as the large number of known causal genes or mutations, and there are currently no effective treatments available. In recent years, cell transplantation using cells derived from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) has emerged as a potential treatment method for RP, but progress has been hindered by technical issues to obtain sufficient numbers of photoreceptor cells at an ideal developmental stage for transplantation, which can survive and form functional synapses with neighboring neural networks within the complex retinal tissue. A groundbreaking report published in 2011 by Mototsugu Eiraku (Unit Leader, Four-dimensional Tissue Analysis Unit) and Yoshiki Sasai (Group Director, Laboratory for Organogenesis and Neurogenesis) of RIKEN CDB cleared this barrier, showing that ESCs could be induced to form photoreceptors as well as three-dimensional optic cups similar to retinal tissues in a 3D culture system, paving the way to consider tissue level transplantation.
Now, in a new study carried out by International Program Associate, Juthaporn Assawachananont, and Deputy Project Leader, Michiko Mandai, and others in the Laboratory for Retinal Regeneration (Project Leader, Masayo Takahashi), they show in that ESC- or iPSC-derived retina sheets transplanted in the eye can survive and integrate with the host retina over an extended period. Published in Stem Cell Reports, their study suggests that, at least in mouse, transplanted retina sheets derived from ESCs or iPSCs are capable of developing structures similar to normal retinal tissues and possibly neural networks, especially when grafts are prepared from younger stage cultures.
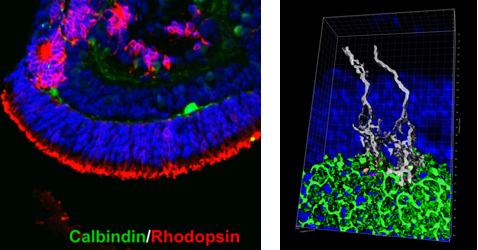
The team first modified the 3D culturing method developed by Eiraku et al. (2011) to generate larger quantities of retinal tissue from mouse ESCs and iPSCs. They tweaked the culture medium by adding a retinoic acid antagonist (RRA) and increasing the concentration of extracellular matrix, which led to a more efficient induction of mESCs and miPSCs into retinal progenitors. Using this modified medium, they examined the optic vesicle-like structures that eventually developed into retina-like structures at differentiation day (DD) 24 composed of an assembly of typical cell types found in the retina such as photoreceptors, amacrine cells, horizontal cells, and glial cells. The differentiation patterns of these cells resembled the development of the mouse retina, and cell cultures younger than DD20 were considered equivalent to embryonic retinal tissues, whereas cultures DD21 and older were similar to postnatal retinas.
The group then examined the feasibility of transplanting grafts of retina-like sheets prepared from ESC- or iPSC-derived optic cup structures induced in 3D culture. Retina-like sheets from various stages between DD11 to 24 were prepared and then transplanted in the subretinal space of mouse models for retinal degeneration, which have lost most of their rod photoreceptors, and the post-transplantation effects were tracked over the course of six months. When retina sheets from younger cultures (DD11-17) were transplanted into host retina, close to 90% of the grafts went on to form a structured outer nuclear layer (ONL) made up of the cell body of photoreceptors. In contrast, in transplants using retina sheets prepared from cultures DD18 and older, most of the grafts (80%) failed to maintain an ONL structure.
Closer examination of the transplanted area revealed that mature photoreceptors in the grafts which formed ONL also possessed the typical outer segment and inner segment. The outer segment had stacked disc-like arrangement when they were nestled adjacent to host retinal pigment epithelial cells, while the inner segment contained mitochondria. Immunostaining experiments indicated that photoreceptors in the grafts showed assembly of their synaptic ends with dendrites of host bipolar cells, resembling normal retinal development. The grafts showed a range of patterns, but those prepared from cultures DD17 and younger showed the most potential to form synapses and maintain an ONL structure.
“Our study is the first to demonstrate that retinal tissue derived from ESCs or iPSCs can be used for transplantation, and the transplanted tissue can survive and integrate with the host retina, even in advanced stages of degeneration,” explains Mandai. “We still need to carry out more experiments to determine the functionality of the transplanted retinal tissue, but we hope to explore the possibility of translating our work into clinical studies for humans, using human derived tissues.”
| Link to article | Transplantation of Embryonic and Induced Pluripotent Stem Cell-Derived 3D Retinal Sheets into Retinal Degenerative Mice |
|---|