
News and Announcements from the CDB
Primitive erythropoiesis during animal development requires stepwise differentiation from pluripotent epiblast cells, which undergo gastrulation to form the three germ layers: endoderm, ectoderm, and mesoderm. Newly formed mesoderm generates hemangioblast cells, the embryonic origins of the blood and vascular system, by initiating expression of early hematopoietic factors. These hemangioblasts then give rise to primitive erythrocytes. A number of studies have noted a delay between the appearance of early hematopoietic markers in mesoderm and that of erythroid lineage-specific globin genes in hemangioblasts. The purpose and the mechanisms at work during this delay, however, remain unclear.
New work by Wei Weng and colleagues in the Laboratory for Early Embryogenesis (Guojun Sheng, Team Leader) now shows that epiblast cells can be made to differentiate directly into the erythroid lineage in vivo through forced expression of five erythropoiesis-related transcription factors and inhibition of the FGF pathway. Published in Stem Cell Reports, these findings suggest a new mode of induced differentiation that bypasses the step-by-step routine seen during development.
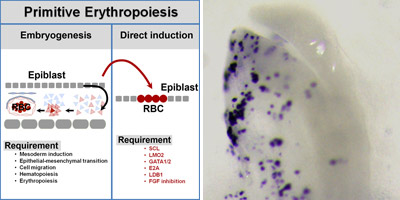
Sheng’s group previously reported that FGF signaling has an inhibitory effect on primitive blood differentiation in chicken. Using an FGF inhibitor to block FGF expression levels in the embryo, this time, Weng and Sheng looked at the effect of FGF activity on early erythropoiesis and on the expression of early hematopoietic factors, SCL and LMO2. A comparison of the effects of FGF inhibition in embryos before or after endogenous expression of SCL and LMO2, suggested that FGF is needed to switch on both of these genes in mesoderm. In FGF-inhibited embryos, they found that exogenous expression of SCL and LMO2 in mesoderm is sufficient to induce robust erythroid differentiation.
Steering pluripotent epiblast cells to differentiate directly into the erythroid lineage proved to be trickier than just inhibiting FGF and turning on two genes. Weng and Sheng searched transcriptomic data for candidate genes with similar expression profiles to SCL and LMO2 and putative regulatory roles during the transition to erythropoiesis. They identified LDB1 and E2A, both of which have been implicated in mammalian hematopoiesis, and are thought to form a complex with SCL, LMO2 and GATA2 to regulate erythropoiesis in vitro.
They made expression constructs of these five genes (SCL, LMO2, GATA2, LDB1, and E2A) and found that when they electroporated the five constructs in combination with FGF inhibition before the endogenous expression of early hematopoietic regulatory genes, they were able to induce strong expression of rho, an erythroid marker, in epiblast. Interestingly, the combination of these five gene constructs and FGF inhibition also induced expression of a gene involved in heme biosynthesis in epiblast, suggesting that this directed differentiation also initiates expression of other essential factors required for primitive erythropoiesis.
“Many groups have tried using one or two key hematopoietic genes to induce erythroid differentiation from mesoderm. What we have done here is to look at inducing erythroid differentiation in mesoderm as well as other lineages,” says Weng. “We have been able to get high efficiency in inducing differentiation in not only mesoderm into a specific lineage, but also in pluripotent epiblast and in ectoderm derivatives that are already less pluripotent than epiblast. But it remains to be seen whether this same efficiency can be achieved in vitro.”
| Link to article | Five Transcription Factors and FGF Pathway Inhibition Efficiently Induce Erythroid Differentiation in the Epiblast |
|---|