
News and Announcements from the CDB
Human pluripotent stem cells (hPSCs) remain in the spotlight for the possibilities they hold out for uses in regenerative medicine. In the lab, hPSCs are famously difficult to handle, as they are extremely sensitive to external stresses such as those caused by experimental manipulations or changes in culture conditions, as these can lead to rapid changes in cell properties or cell death. One especially stressful technique is cryopreservation, which is characterized by low recovery rates of hPSCs after the thawing processes. Further technical developments to cryopreservation are needed to improve cell recovery rates, but any such method must also be simple, efficient, and inexpensive.
In a new study focused on hPSC preservation, Teruo Akuta, Keitaro Imaizumi, and colleagues in the Laboratory for Stem Cell Biology (Shinichi Nishikawa, Group Director; the Nishikawa lab closed in March 2013) have developed a new slow-freezing method that can be used to store large quantities of hPSCs easily and cost-effectively. Published in PLoS One, they took a commercially available medium used for the cryopreservation of cells from cord blood and bone marrow, and optimized it specifically for hPSCs. The group also determined the most effective dissociation solution to be used in combination with the modified medium. The study was carried out in collaboration with the laboratory of Shin Kawamata (Vice Director, Foundation for Biomedical and Innovation).
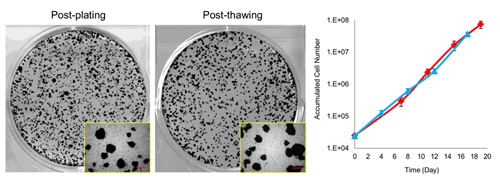
The two methods currently used for hPSC cryopreservation are vitrification and slow-freezing. Vitrification, which requires special skills, uses liquid nitrogen to quickly freeze the cells placed in a medium with high concentrations of cryoprotectants and is not suited for the cryopreservation of large quantities of cells. The slow-freezing method, where cells are suspended in cryopreservation media and then slowly allowed to freeze in a deep freezer overnight, is simpler and can be used to cryopreserve large quantities of cells. The efficacy of any form of cryopreservation depends on the dissociation step carried out just prior to freezing, in which the colonies are broken up into small clusters, as the surface area of clusters bathed directly by cryoprotectant varies by the size of cell clusters following dissociation.
The group first examined five known dissociating solutions to determine which is most suited for the cryopreservation of hPSCs, and the recovery rates of cells treated with different solutions were examined post-cryopreservation by staining them with alkaline phosphatase (ALP). They found that treatment with Pronase/EDTA resulted in relatively small, uniform cell cluster sizes, and yielded the highest recovery frequency (44%). Trypsin/EDTA also generated small cell clusters, but the cell recovery frequency was less than half of that seen for Pronase/EDTA; other dissociation solutions tested produced large variations in cell cluster size and cell death after thawing.
Given its favorable properties, Imaizumi et.al. next looked to develop a hPSC-specific cryopreservation medium compatible with Pronase/EDTA. They prepared a panel of new cryopreservation formulas by changing the concentration of reagents in a popular commercially available freezing medium, and adding other known cryoprotectants. When they compared the efficacy of cell recovery after thawing, they found that the addition of ethylene glycol (EG) yielded the highest post-thaw recovery rates. They tweaked the formula further to identify the optimal concentration of reagents plus EG. The most efficient formula, CP5E, consisted of a mix of 6% hydroxyethyl starch, a natural cryoprotectant derived from plants, 5% dimethyl sulfoxide and 5% EG.
The group next tested the optimized cryopreservation medium in combination with the Pronase/EDTA solution on different hiPSC and hESC lines, and found to yield recovery frequencies of over 80%. When the group evaluated the cells before and after cryopreservation, they found no significant differences in cell properties. hPSCs subjected to the new cryopreservation method maintained their proliferative capacity and pluripotency on thawing, and showed no structural chromosomal abnormalities.
A major advantage of the hPSC cryopreservation medium developed by Imaizumi et. al. is the absence of animal and protein components. By simplifying the composition, they were able to avoid such risks as lot-to-lot variation and infections from pathogenic agents, which can make bovine serum albumin or serum solutions problematic. The coupling of this new method with a novel dissociation agent has also made cryopreservation of hPSCs more efficient and straightforward.
“The increasing demand for use of hPSCs in drug discovery and regenerative medicine has made it essential (for scientists) to establish safe, simple, efficient, and inexpensive methods for handling these cells,” says Nishikawa. “We hope that this study will lower some of the technical barriers to starting stem cell research, and help accelerate both basic and applied studies in the field.”
| Link to article | A Simple and Highly Effective Method for Slow-Freezing Human Pluripotent Stem Cells Using Dimethyl Sulfoxide, Hydroxyethyl Starch and Ethylene Glycol |
|---|