
News and Announcements from the CDB
Age-related macular degeneration (AMD) is a disease of the eye that affects visual acuity in the elderly. Degeneration of the retinal pigment epithelium (RPE), a pigmented cell layer in the eye that supports and supplies nutrients to the photoreceptors, is a major pathogenic factor in AMD. Of the major forms of this disease, neovascular AMD, commonly known as ‘wet-type,’ is more prevalent in Asian populations. There is currently no cure for wet-type AMD, but several groups are exploring the possibility that pluripotent stem cells may be a useful source for generating functional RPE for use in transplantation. The efficacy and safety of such stem cell-derived tissue, however, needs to be tested extensively before studies in human can begin.
A new preclinical study by Hiroyuki Kamao and colleagues in the Laboratory for Retinal Regeneration, reports that RPE cell sheets generated from induced pluripotent stem cells (iPSCs) show an excellent efficacy and safety profile in animal transplant models. Published in Stem Cell Reports, this work lays the groundwork for the world’s first pilot clinical study on transplanting hiPSC-RPE cell sheets to AMD patients.
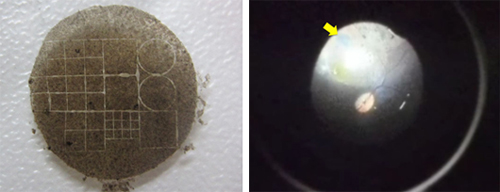
Kamao et al. produced RPE cell sheets that did not have an integrated artificial scaffold, but could withstand the manipulations required for transplantation procedures. They were able to do so by growing the stem cell-derived RPE cells on a degradable temporary collagen scaffold. Several groups have previously reported using artificial scaffolds to generate RPE cell sheets; however, these scaffolds run the risk of causing inflammation or creating physical barriers that limit nutrient flow from choroid to photoreceptor cells. The group confirmed that, in vitro, the cell sheets expressed RPE markers and basement membrane components and had formed functional tight junctions exhibiting polarized secretion of growth factors similar to the activity of native RPE.
Kamao and colleagues next tested the function of these hiPSC-RPE cell sheets in vivo by transplanting them into the subretinal space of immunosuppressed three-week-old RCS rats, a model of inherited retinal degeneration. Using electroretinogram (ERG), they watched for changes in photoreceptor activity at six weeks post-transplant, and three weeks later measured the thickness of the outer nuclear layer (ONL), where the photoreceptors reside. Compared to control and sham-surgery animals, rats that received transplants of either hiPSC-RPE cell sheets or cell suspensions maintained the ONL and showed significant restoration of ERG responses, suggesting that the hiPSC-generated RPE cell sheets are functionally similar to native RPE.
o confirm the feasibility of using the iPSC-derived grafts in clinical studies, the group tested and compared the immunogenicity of autologous and allogeneic grafts in cynomolgus monkeys. To verify whether immunogenic responses are triggered by allogeneic or autologous grafts in vivo, Kamao transplanted cell sheets derived from a single donor monkey into the subretinal space of four non-immunosuppressed monkeys—the donor and three MHC-incompatible animals—and observed them for one year. The group found that tissues surrounding the allografts showed typical signs of graft rejection, while those surrounding the autograft showed no signs of rejection. The transplanted cells also did not contribute to tumor formation, which has been of the major concerns for pluripotent stem cell-derived cell products. A final comparison between the transplantation of cell suspensions and cell sheets indicated that cell sheets offer several advantages; they can be transplanted directly at the lesion site and monitored easily, as they remain anchored in place.
“Safety is of the utmost importance, and these animal experiments show that autologous grafts are not rejected and non-tumorigenic. Tumor formation in the eye is relatively rare to begin with, and at least for AMD, transplantation does not require a large number of cells, so quality control is relatively simpler,” says Takahashi. “We can also detect subtle changes in the eye, so in the event that any abnormalities develop, we can catch them at an early stage. All of these factors make AMD a good target for the world’s first clinical study of iPS cells.”