| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
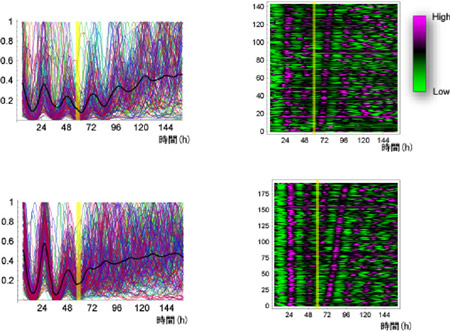
Reckoning that a photoinducible system in vitro would allow them to study singularity in a simpler, more controlled fashion, Ukai transfected melanopsin, which is normally only expressed in retinal cells, into mouse fibroblasts, exposed them to a 6-hour pulse of light and measured the effect on biological clock activity by monitoring changes in the expression of PER-2, a clock gene intrinsic to these cells. Melanopsin-positive cells exposed to a light pulse showed changes in both the phase and amplitude of PER-2 expression, while untransfected cells did not, indicating that the melanopsin protein had made the fibroblasts photo-responsive. A series of tests using light pulses delivered at different time-points yielded one critical pulse capable of inducing singularity in vitro. They looked next at the individual cells to decipher how singularity manifests itself, and found that although each cell generally maintained their intrinsic rhythmicity, but that they cycled out of step with other cells. This stands in contrast to several previous studies that had suggested that multi-cell singularity is the result of loss of rhythmicity in individual cells. So whether desynchronization would be observed in vivo remained an important question. The team entrained rats in a laboratory under conditions known to induce singularity in humans, and studied a section of their brains, the suprachiasmatic nucleus (SCN), known to be the master of the body’s biological clocks. The expression of clock genes Per1 and Per2 showed a dramatic decrease in total amplitude, as well as a pattern of expression that correlated to the desynchronization seen in vitro, suggesting that a similar mechanism may be at work in vivo as well. The model is not without its caveats, though, as the SCN cells appear to lack cell-cell coupling and the induction of singularity in vivo occurs indirectly, via the effect of light pulses on retinal neurons, while the effect is direct on the melanopsin-transduced fibroblasts in vitro. Nonetheless, this work by Ukai, Kobayashi et al., represents an important new insight into a question now more than 30 years old. The phenomenon of singularity is of fundamental interest not only for its biological significance, but for the role it plays in some forms of sleep disturbance inked with late-night exposure to artificial, an increasingly common occurrence in the modern world. “It’s interesting what this has revealed about how well-balanced a system the biological clock is,” say the authors. “The individual cells keep each other in a steady cycle that is robust in the face of most environmental stimuli.”
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |